July 25, 2024
Quality in Quantity
When new medicines come onto the market that promise relief or even a cure for a large number of people, large quantities have to be produced quickly—in the highest quality and purity. HMI systems such as those from Pepperl+Fuchs are used to ensure that personnel in the large-scale production of pharmaceuticals always have an overview of all information and can document work steps.

Pharmaceutical research has repeatedly achieved success in combating diseases that affect many people worldwide, which has offered great benefits to patients and healthcare systems. Following the rapid development of COVID-19 vaccines, weight loss injections for the treatment of obesity are the latest example of such a breakthrough. Obesity is one of today's most prevalent health issues, especially in the Western world. Various remedies have already been used in recent decades as an alternative to invasive procedures, but they have often failed to achieve the success promised. These weight loss injections offer a new option. Several pharmaceutical companies have succeeded in developing an active ingredient that not only helps patients with type 2 diabetes, but, at an adjusted dosage, has also been used to treat patients with obesity for some time.
Once a week, the patient injects themselves with the drug, which reduces the appetite to such an extent that the patient feels noticeably less hungry—and ultimately loses weight. There should be relatively few side effects, meaning a real trend has already developed. Especially in the USA, where the weight loss injections were first approved for obesity treatment, there was a wave of sales that gradually spilled over into Europe. Manufacturers reported initial supply bottlenecks shortly after the market launch and therefore are now planning to significantly expand production capacities for the active ingredients due to the huge global demand. Stefan Sittel, pharmaceutical industry expert and Business Development Manager HMI at Pepperl+Fuchs, is aware of the importance of medicines that can help a large number of patients worldwide: "Producing blockbuster drugs such as urgently needed vaccines and the new weight loss injections presents extraordinary challenges for pharmaceutical companies and their suppliers of basic, auxiliary, and pre-products. Hundreds of millions of doses have to be produced every year—with the highest levels of quality and purity." Production sites are often expanded or newly constructed as early as the approval stage by relevant authorities—such as the FDA in the USA or the EMA in the EU—within a short period of time in order to scale production as quickly as possible and meet demand.

Extraordinary Challenges in Every Respect
It is not only the large quantities required that pose challenges for companies in the pharmaceutical industry, but also the laws and regulations of this highly regulated sector. This means that quality and purity must be ensured for each individual production step and perfectly documented, and that corresponding data must be stored reliably. Batch production is therefore still common in the pharmaceutical industry to consistently monitor and guarantee production processes and quality. It is clear that pharmaceutical companies benefit in particular from the digitalization and automation of as many production steps as possible. As a result, HMI systems such as those from Pepperl+Fuchs are increasingly being used in pharmaceutical production. Using standard Ethernet communications, the systems allow access in the field to the process control system for monitoring and controlling production processes at any time, and access to the manufacturing execution system (MES) for processing and documenting recipe steps. "State-of-the-art, thin-client-based devices establish a connection to remote, virtualized SCADA applications and the MES and reproduce their individualized user interface," explains Sittel. "Depending on the process step and operating location within the pharmaceutical production, the HMI systems must be suitable for different ambient conditions. The Pepperl+Fuchs portfolio is precisely geared toward these diverse requirements." After all, the tablets or injections used by patients at the end of the value chain have been through a production process that is by no means simple.

Easy to Use, Complex to Produce
For a number of years now, the trend in the pharmaceutical industry has been toward biopharmaceutical drugs, i.e., drugs produced using genetic engineering, which include current weight loss injections. Biopharmaceuticals are based on genetically modified yeasts, bacteria, or cells, which are initially multiplied in closed containers, and the desired active pharmaceutical ingredient (API) is produced via fermentation. "These stages of production are usually carried out in pharmaceutical cleanrooms. Purity and protection against contamination are the top priority—thorough cleaning is carried out after each batch," explains Sittel.
The active ingredient is then subject to further chemical and physical processing. The HMI systems used must meet correspondingly stringent requirements for cleanability and chemical resistance, and comply with GMP (Good Manufacturing Practice) guidelines. Pepperl+Fuchs supports these demanding production environments with a range of operator workstation versions and appropriate peripheral devices. "Our VisuNet series are easy to clean with their rugged stainless steel housings, have no inaccessible gaps or surfaces where deposits can collect, and are able to withstand conventional, aggressive cleaning agents," says Sittel, summarizing the broad portfolio. "The VisuNet FLX series is designed for use in pharmaceutical cleanrooms and also offers versions that can be used in ATEX/IECEx Zone 2/22 hazardous areas."
The VisuNet GXP series is available for production environments with explosion protection requirements in accordance with ATEX/IECEx Zone 1/21. All industrial monitors are available with a touch screen behind glass, in addition to the combination with an antibacterial membrane keyboard with integrated cursor control (touch pad, trackball, or joystick). The touch screen can also be operated while wearing gloves and is ideal for the demanding ambient conditions found in pharmaceutical production.
A Range of Options
The modular design of Pepperl+Fuchs thin-client-based monitors and operator workstations not only offers a high degree of flexibility for customized assembly in production, but also long-term investment protection. In the event of new requirements, individual components such as the CPU can therefore be replaced on-site in a modular manner.
In addition to the versions for wall, ceiling, or floor mounting, operator workstations for panel mounting are used for processing the active ingredient by filtering or dosing, which can be integrated directly into the large machines or with special installation housings in pharmaceutical walls. Panels in the VisuNet FLX series are ideal for this purpose and always provide the operator a view of all the necessary information—right where it is needed. The VisuNet FLX is also the first choice for subsequent processing of the active ingredient into a tablet, juice, or injection. "With paperless production on the rise, we are increasingly seeing demand for installation of two monitors on one operator station: One for automation, one for paperless production using MES," says Sittel. "We are therefore offering duplex solutions to our customers to exactly meet this demand." For efficient support in uncommon or long-lasting process steps, all operator workstations are also available as a mobile unit on rollers.

To prevent unauthorized access to the operator workstations and to identify personnel in accordance with regulations (FDA CFR 21 Part 11 or EMA Annex 11), today RFID readers are mainly used in the operator workstations, which operators can use to log on. A PIN or password must be entered using the keyboard as the required second step of identification. Together with the security mechanisms of Pepperl+Fuchs thin client firmware, the required level of security in pharmaceutical production is ensured at all times.
Efficient Thin Client Management
VisuNet RM Shell firmware is pre-installed on all Pepperl+Fuchs thin clients. The firmware is based on Microsoft® Windows 10 IoT Enterprise LTSC 2021 and simplifies the setup of thin client devices and their integration into virtualized and conventional PC-based process and manufacturing systems. In addition to the latest Windows security features and password-protected access control, the firmware includes numerous integrated security mechanisms, including hybrid user rights management that prevents unauthorized access.
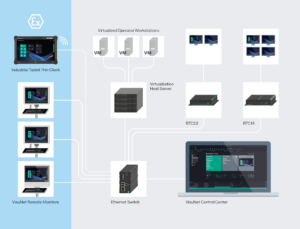
The VisuNet Control Center thin client management software ensures highly efficient management of thin clients in pharmaceutical plants. Production managers, OT managers, and support staff can connect remotely to any operator workstation with VisuNet RM Shell, monitor the device status, and view the screen content, allowing them to configure, update, and manage all thin clients from a single PC in the network. Updates can be applied to all thin client devices simultaneously, ensuring that they are all up to date with respect to security. "Our software eliminates the need to enter hazardous areas and cleanrooms to set up and maintain remote monitors," says Sittel, summarizing the benefits. He adds: "Our thin clients also support other common thin client management systems such as ThinManager® and IGEL®, so that customers using these systems also benefit from our customized hardware."
From Laboratories through Cleanrooms to Zone 1/21
To enable users to also access controllers and systems for laboratories, media infrastructure, and building technology and manage them via the VisuNet Control Center, Pepperl+Fuchs offers small industrial box thin clients (BTCs) without a monitor. In a rugged aluminum housing with an extended temperature range, these BTCs are optimized for 24/7 industrial operation. Depending on the version, the BTCs enable the connection of two to four standard Ultra HD monitors.
As a partner for HMI solutions in the pharmaceutical industry, Pepperl+Fuchs offers an end-to-end thin client portfolio and HMI systems for all areas of pharmaceutical production up to Zone 1/21. "For cases where modular standard products do not meet individual requirements, our experts at Pepperl+Fuchs Solution Engineering Centers (SECs) develop and produce customer-specific solutions adapted exactly to the application," explains Sittel. "All conforming to GMP."